Best Of The Best Tips About How To Write Consent Form

Start with a descriptive title.
How to write consent form. Follow these steps to write an effective consent form. Begin by introducing yourself and the purpose of the. The consent form should be written in the second person.
From rags to riches: The procedures for obtaining consent should be documented on the consent tab of the. To begin, choose from a selection of sample consent forms and customize them easily to suit your specific needs.
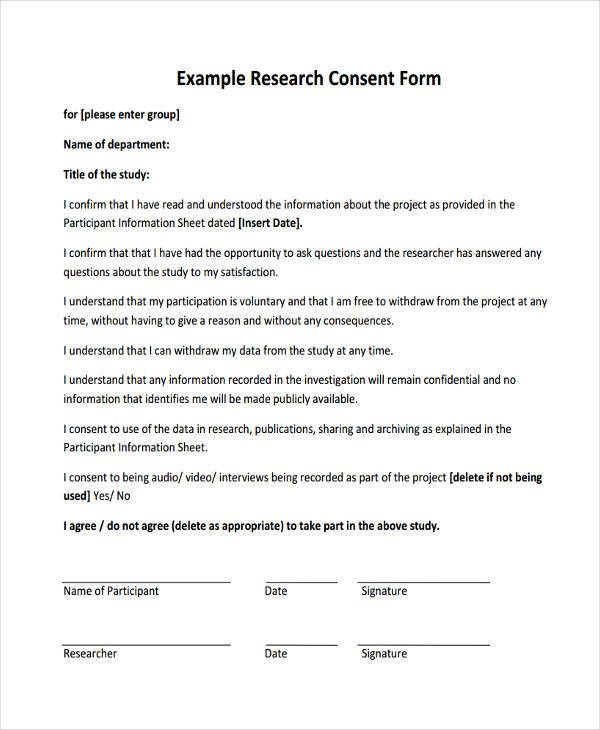
A study description and purpose. Here’s a basic format for informed consent that can be customized for specific research studies: Below are some useful tips on writing a consent form for your proposed subject population.
All four elements of consent are equally important, namely; Wendy gough, bunkyo gakuin university; Explain the purpose or reason for which you are providing consent.
Choose a sample consent form to customize. A consent form template for ux research studies what is an informed consent form? Salutation or greeting:
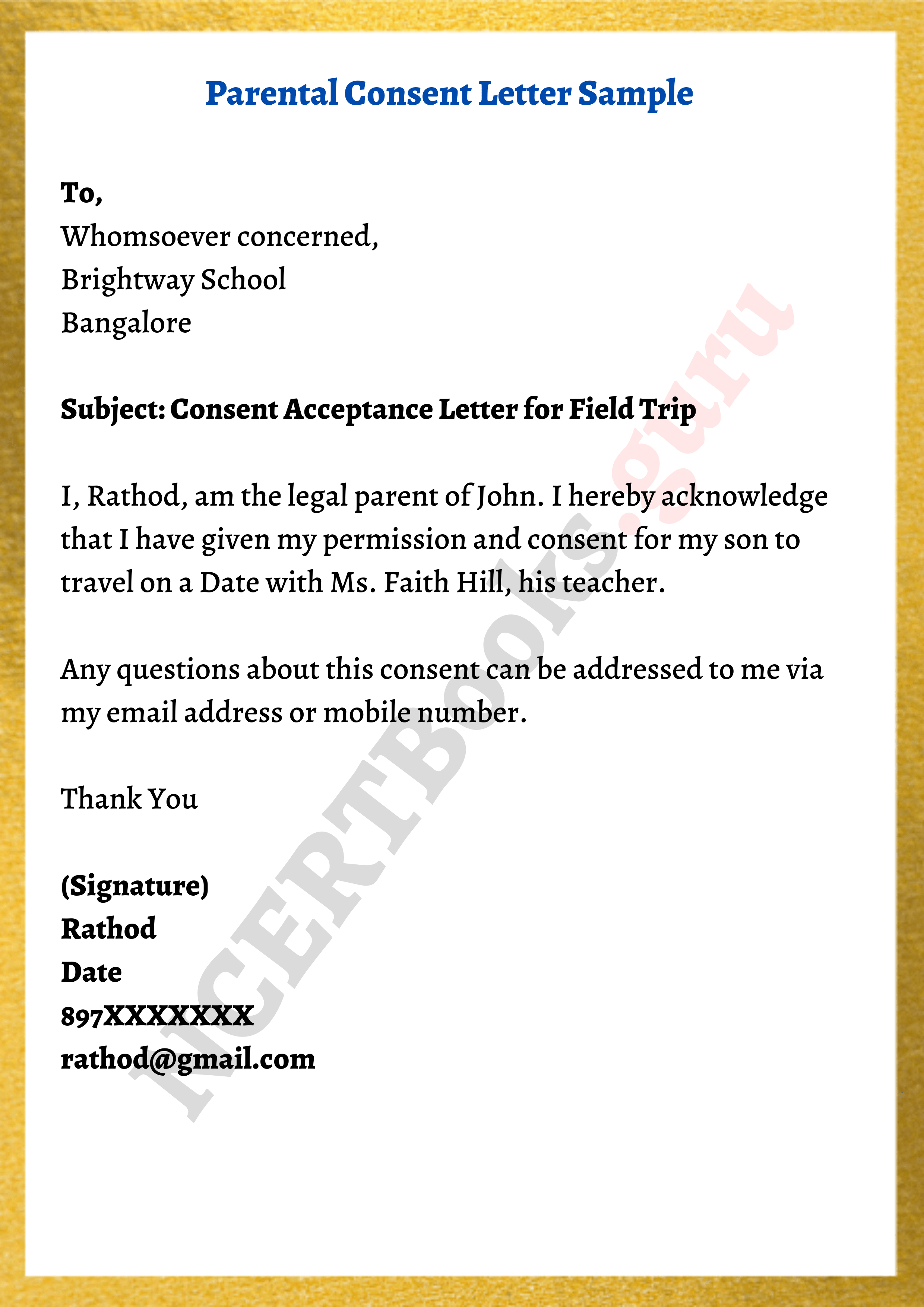
It says that ai systems that can be used in different applications are. Guidelines for writing a consent letter. Dear sir/ma’am, body of the letter:
Recruitment documents help people make informed choices about whether to participate in a research study. Informed consent documents should be written in plain language at a level appropriate to the subject population, generally at an 8th grade reading level. In april 2021, the european commission proposed the first eu regulatory framework for ai.
Briefly explain why the study’s being conducted. You should at the very least describe: How to make compliant gdpr consent forms (with examples) if your business is subject to the requirements outlined by the gdpr, you may use.
Describe explicitly intents to make data available for. Your research and your team. A consent form gives written permission to another party to perform an activity or host an event, indicating that the signatory.
Let’s use a dental consent form as our example document here. Kick off your consent form by outlining your study's purpose and objectives. Researchers who conduct studies involving human participants should always obtain.
![FREE Consent Form [PDF, WORD]](https://images.sampleforms.com/editor/wp-content/uploads/2020/07/Consent-Form.png)